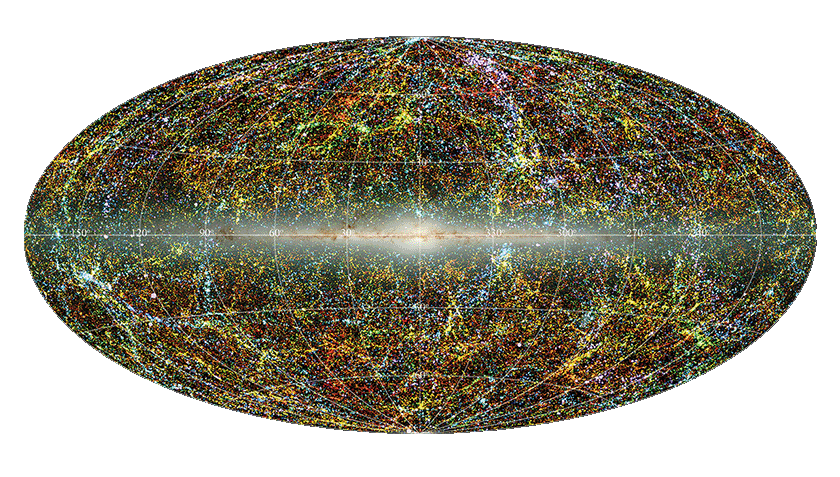
The discovery of the cosmic microwave background in 1965 changed the way the universe was viewed. Originally thought to be caused by proximity to New York City, it representes the remnants of the big bang. A small portion of the static or “snow” seen on an untuned analog television screen is a visual pattern of the radiation. This radiation is the leftover glow from the Big Bang, a faint echo that permeates the entire universe.
The best explanation I could find for the Big Bang is that there was nothing, and then, in the next moment, space came into existence. Simultaneously, every point in space began moving away from every other point in space. Go figure!
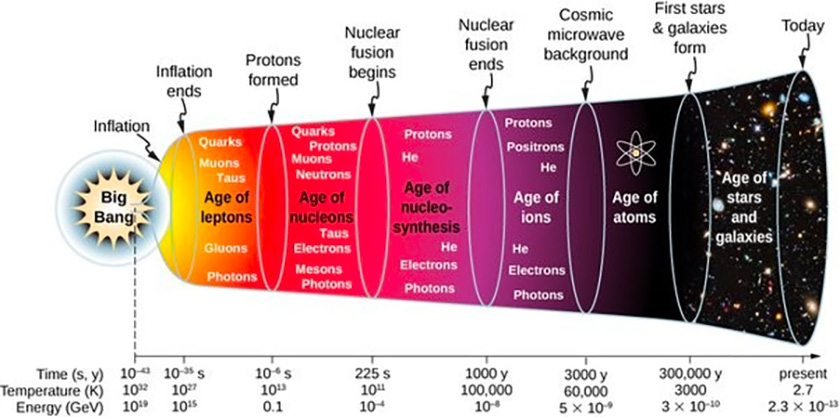
One thing is clear: We live in a universe of energy. The higher the energy level for a closed system, the hotter it gets. The ‘temperature‘ of the Big Bang is thought to be around 1032 °K. That is a ten with 32 zeros after it:
100,000,000,000,000,000,000,000,000,000,000 °K
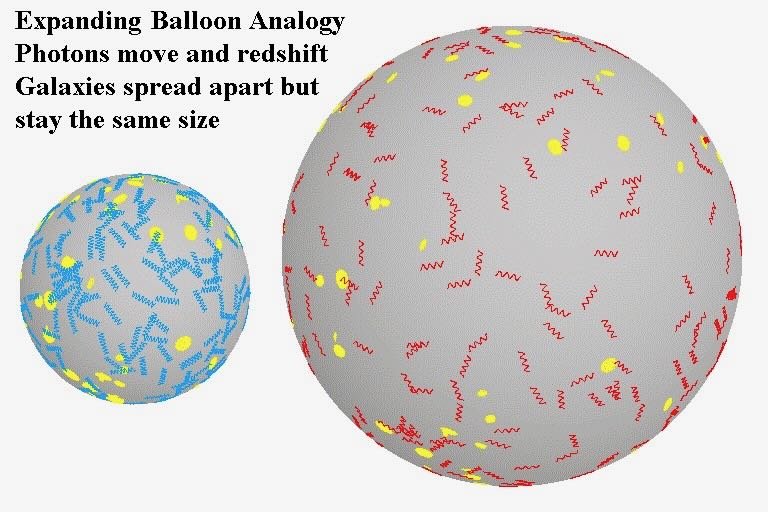
… for the briefest of an instant, it is thought that things cooled down quickly, and space expanded liked the balloon analogy (above).
May the Force be with Us
Energy coalesced into the first proto-matter, comprising strange wavicles (aka particles) called quarks, gluons, and leptons. These particles were influenced by powerful forces operating at infinitesimal distances. Quarks and anti-quarks of different types, together with an ever-changing number of gluons (the massless force carrier of the strong force), combine to form nucleons, which are known as protons and neutrons, by colliding at or near the cosmic speed limit.
We know know that quarks get their intrinsic mass from interaction with the Higgs field. This was confirmed only recently by experiments at the Large Hadron Collider at CERN. However, this mass only accounts for a small percentage of the mass in the universe.

Because quarks are confined to a tiny volume (the radius of a proton) by the strong force, Heisenberg’s Uncertainty Principle dictates they must move at incredibly high speeds—nearly the speed of light. This massive kinetic energy manifests as mass. This makes up over 99% of the total mass of the universe.
As the universe expanded and cooled, these particles interacted with each other and other particles like electrons and photons— these being the constituent components of the electromagnetic force. Things started to clear up.

Approximately 300,000 years after the Big Bang, the universe cooled down enough for electromagnetic radiation, including visible light, to fill it. The Cosmic Microwave Background (as shown at top) represents the farthest we can look back in time.
The Age of Matter
The stage was set. Everything that comes afterwards, including amazingly complex self-organizing stuff that forms the basis for living organisms, is based on the result of this cosmic inflation.
E equals f h,
Matt Strassler – Waves in a Impossible Sea
And E equals m c squared;
From these seeds, the world
As the hadrons interacted with other particles like electrons, they formed an equivalent number of hydrogen atoms, plus a few helium and lithium atoms. The amounts were small compared to the ever-expanding volume of the universe.
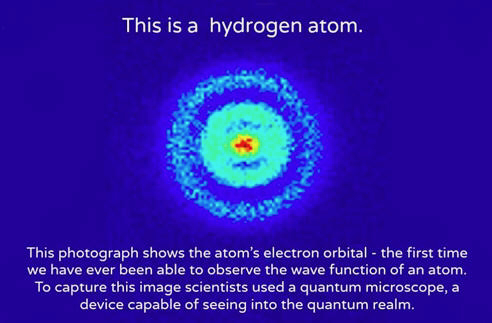
There were so many of these hydrogen atoms that their mass affected spacetime by bending it. We call this force gravity.

These atoms started to clump together and became so big and hot that they ignited in nuclear fusion. A star was born, so they say.
Stars are fascinating. They are born in dense clouds of gas and come in all sizes; depending on their size, what happens when they run out of hydrogen differs.
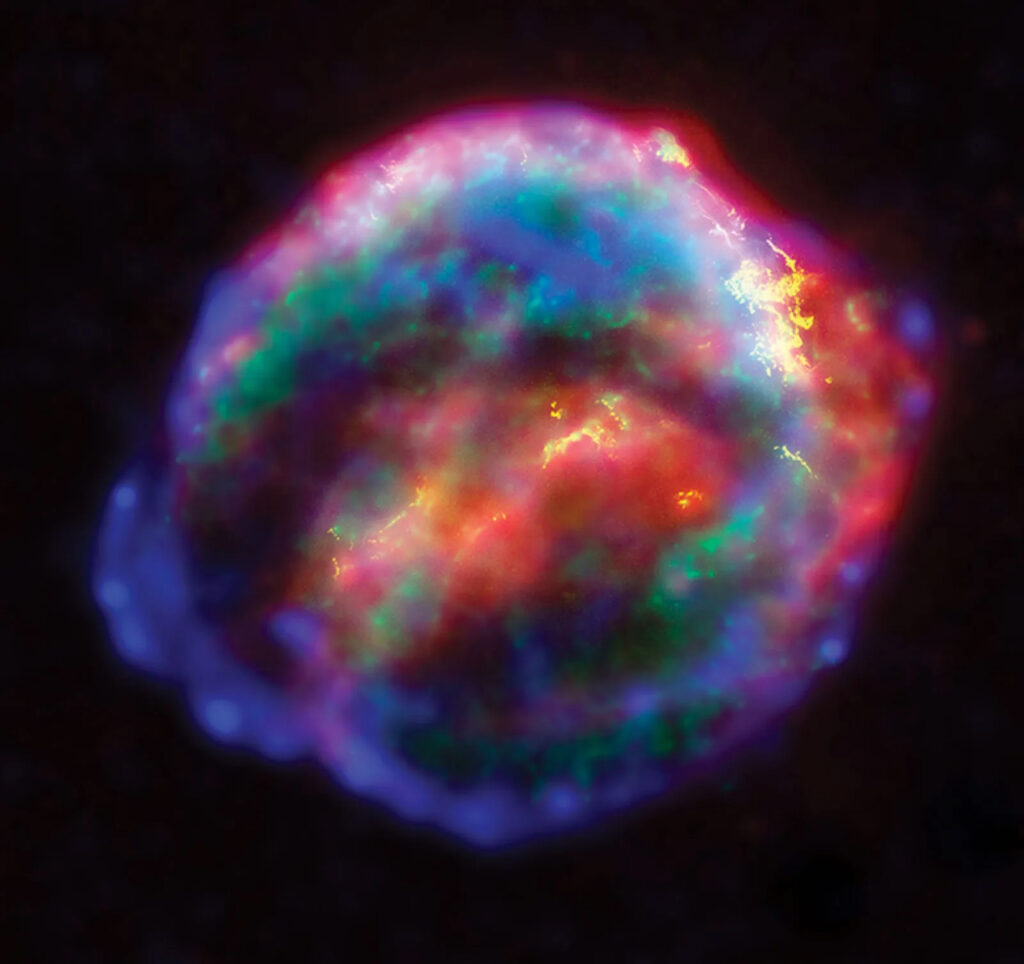
As these early stars reached the end of their lifetime, they died in different ways.
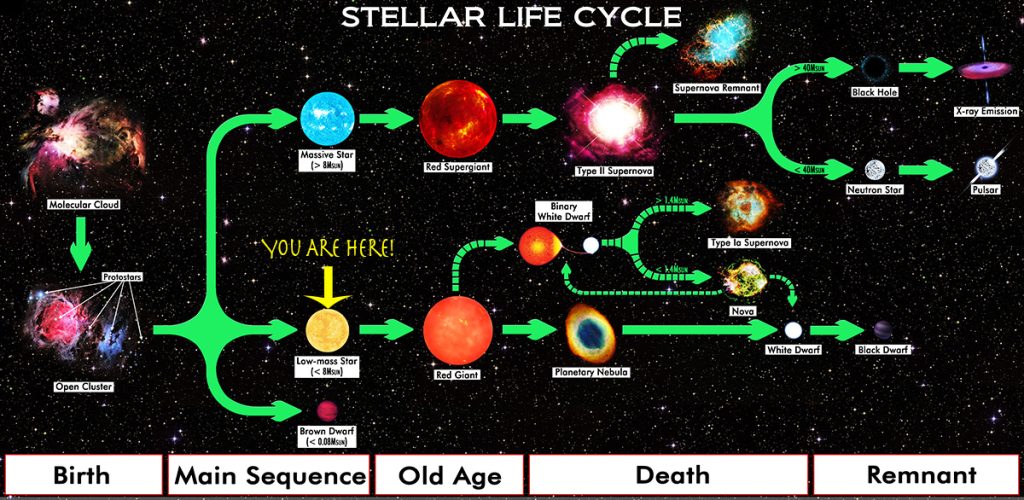
Smaller stars like our Sun create oxygen and other ‘metals’ like carbon and silicon—everything up to iron, the most stable element, as they age. More giant stars explode in what’s called a supernova. In the explosive death throes of these stars, heavier, exotic elements like Uranium are formed. As a star dies, it sheds these elements to become part of the dust and gas clouds that birth new stars.
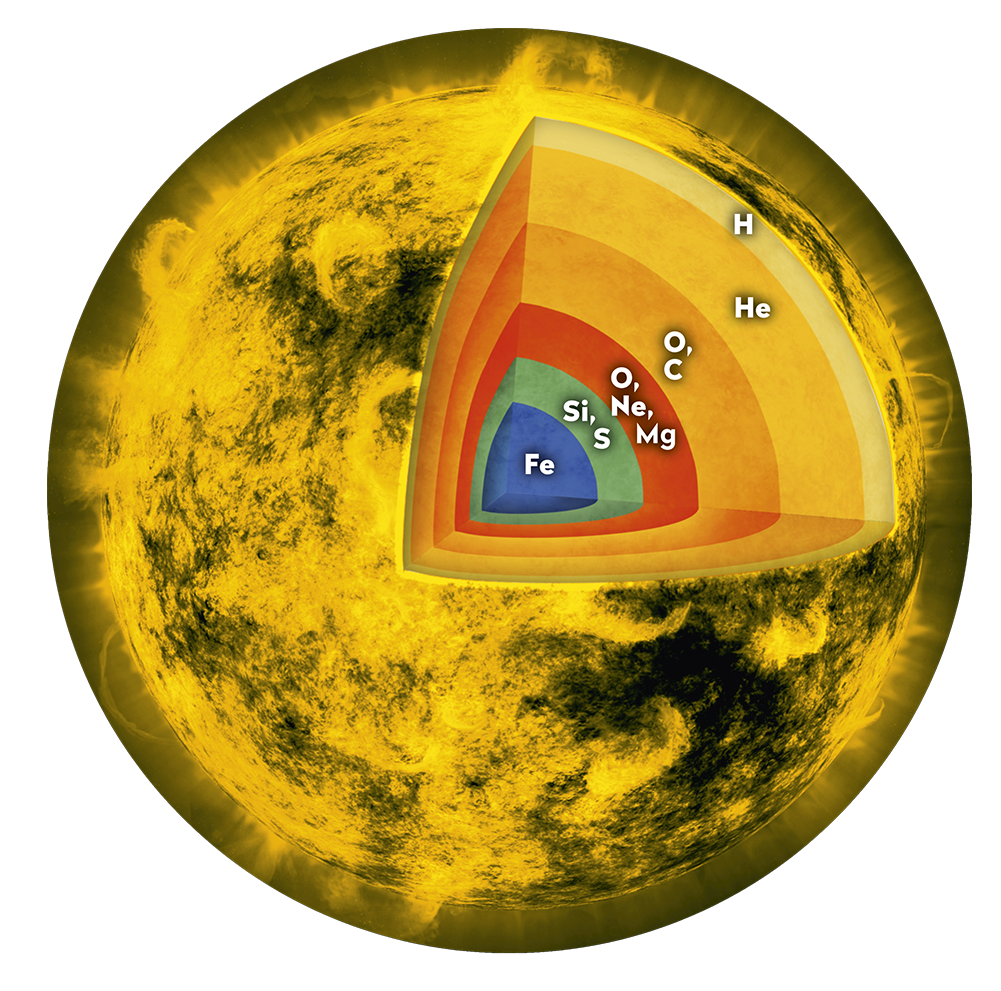
There are 4 × 1027 hydrogen atoms in the human body, well over half the total number of atoms. There are also about 2 x 1027 oxygen atoms in the human body. Every one of the oxygen atoms was made in the death of a star sometime, somewhere, a long, long time ago. We all know that two hydrogen atoms combine with one oxygen atom to make water. So roughly 2 x 1027 of the molecules in our body are water. The next most common element is carbon, with about 8 x 1026 atoms. So we are, in fact, mostly bags of water. Thank you, Mother Ocean.
The the atoms in a human body are far, far more numerous than the sand grains on all the beaches of the Earth; than the leaves and needles on all the trees of the Earth’s forests; than the number of seconds since the turmoil of the Big Bang; than the heartbeats of all the people who have ever lived upon our planet; than even the stars—not just the ones you can see in a dark sky, not just the hundreds of billions in our Milky Way galaxy—far more numerous than all the stars in all the galaxies across the entire visible universe.
Matt Strassler, Waves in an Impossible Sea
The Quantum Foam
Quantum field theory, one of science’s most elegant and precise concepts, shows us that all forces and matter are manifestations of the continuous motion of quantum fields.

In seemingly empty space, quantum particles pop in and out of existence, dancing in the presence of complex quantum fields. Hot hydrogen gases are interlaced with the more complex matter. Cosmic rays bombard the matter, creating new elements. Gravity collapses the dust into a new star, and the cycle continues.
Everything is always moving. Atoms are moving. Energy waves are moving (whether it’s radio waves or microwaves or even the waves of light that appear as different colors). The planets and stars and the galaxies are spinning and the universe itself is constantly expanding at an even quicker rate.
Some smart guy on the Internet
Welcome to Earth
All this is happening on the unimaginably grand scale of the cosmos. Untold number of galaxies housing millions and millions of stars. We know this because of our incredibly large craniums that many, if not most, of these stars have one or more ‘exoplanets.’
“Mostly Harmless”
The Hitchhiker’s Guide to the Galaxy entry for Earth, updated by Ford Prefect
And as the cosmic roll of the dice goes, we ended up on a rare, but likely not unique, planet with a liquid metal core and vast quantities of water. Early in our planet’s lifetime, another, smaller chunk of cosmic debris crashed into it and left us with a moon. The spinning magnetic core provided a safety net guarding against solar radiation, and the water provided, among other things, a critical component of the evolution of life. The Moon gave us something to be amazed by and created tides to help churn everything together.
Motion, as it turns out, is always relative to a frame of reference. Big-brained Einstein stunned everybody with this fact over a hundred years ago. And as it turns out, the fastest anything can go in our universe happens to be the speed of light.
Relative to the CMB (see above), it turns out we are moving about 1.4 million miles an hour, about 0.1% of the speed of light. While this is going on, the Earth rotates about one thousand miles per hour on the surface at sea level; the Earth rotates around the Sun at 68,000 miles per hour; and the Sun rotates around the center of the Milky Way’s giant black hole Sagittarius A* at half a million miles per hour.
Truly, nothing is standing still no matter where you look in this amazing universe we are so incredibly lucky to experience.
The Genie 🧞
All behave the laws of physics in the most extraordinary ways. Now add the Second Law of Thermodynamics, which essentially states that you can’t put the genie back in the bottle (or perhaps you can’t un-break an egg). The star stuff got really complex. I mean, it’s really complex. Things really start to get interesting.
One thing leads to another. Most of what resulted was disregarded. An infinitesimally small number of things would play out. It was chaos with a purpose.

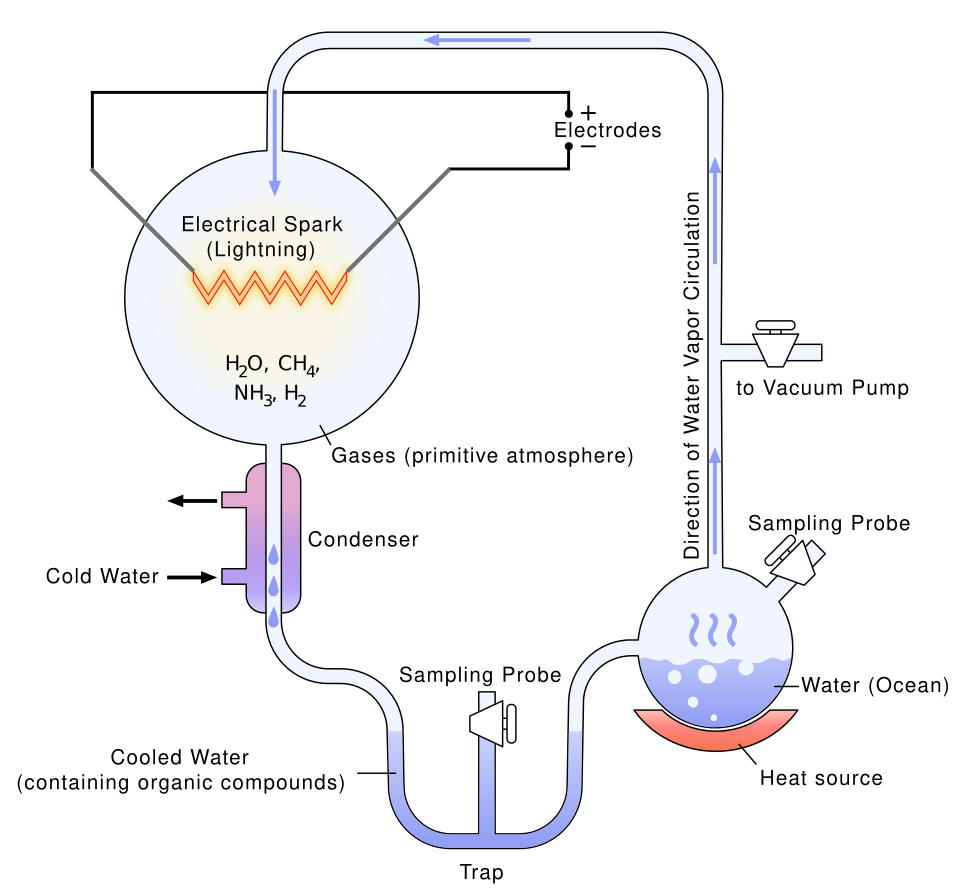
Stanley Miller, a bag-of-mostly-water creature as well as a brilliant scientist with an all-too-conventional name, had an audacious idea. He aimed to recreate the conditions believed to have existed on Earth shortly after its formation, approximately 4.5 billion years ago. Despite facing skepticism and resistance from others, Miller remained resolute in his pursuit of this groundbreaking experiment. He proceeded with unwavering determination, regardless of the doubts cast upon him. He discovered that the key ingredients of life were indeed created by this outrageous idea!
Other scientists like Stanley believe that the essential components of life may have originated from outer space. In recent years, some of them did some big-brained engineering, launched a rocket into space (just up the street from here), and discovered the potential ingredients for life on asteroids.


Remember that molten metal core? Most scientists now believe that life evolved in hydrothermal vents deep in the ocean’s bottom. Cold seawater, near freezing, surrounds the vents, which can reach temperatures of up to 750 degrees Fahrenheit. These conditions could very well exist elsewhere in the solar system, on moons like Europa.
What Does It All Mean?

What ever the case, in a most mysteriously joyful act of serendipity, one of the results was a Mostly-Bag-of-Water Ape Descendant. It had no fur but, instead, sweat glands so it could run for long periods without tiring out like its cousins. Opposable thumbs allowed it to grasp tools. It couldn’t see, hear, or smell quite like the other creatures, but it stood upright and could see danger coming. Most importantly, a large cranium housed a relatively large brain capable of amazing thought – but needed some time to become fully functional. When it did, as we have shown, amazing things started to happen.
Far out in the uncharted backwaters of the unfashionable end of the western spiral arm of the Galaxy lies a small, unregarded yellow sun. Orbiting this at a distance of roughly ninety-two million miles is an utterly insignificant little blue-green planet whose ape-descended life forms are so amazingly primitive that they still think digital watches are a pretty neat idea.
Douglas Adams, Hitchhiker’s Guide to the Galaxy

Eventually, these Ape Descendants rose to the very top of the apex of all the other living creatures made from star stuff. They invented the wheel. They invented God. They had wars and babies. They split the atom. One of these wars, the last one that pretty well affected everyone, was World War II. As these Ape Descendants liked to call themselves, Humankind had come a long way in killing each other. Things were dire.
Decades earlier, Humankind had finally thrown off the yoke (a bit anyway) of religious trappings that discouraged thinking outside the box. They discovered, among other things, that matter and energy are really the same thing. It helped to explain a lot.
Because things were so dire, some of them got together and determined that they could use this fact to build a weapon of unheard-of power. All you needed was some tiny fraction of star stuff. Specifically, one created in minute quantities in these supernovas, or better yet, in a neutron star. A neutron star is 6 miles in diameter and weighs nearly twice as much as the sun. Wow. This stuff is called Uranium after the Greek god Uranus – the god of the sky. Double wow.

Sure enough, this super weapon ended the war and drop-kicked us into the modern age we live in now. This was never more evident than in the little, mostly unknown state of New Mexico.